Zoning
Crystal zoning is a texture developed in solid-solution minerals and characterized optically by changes in the color or extinction angle of the mineral from the core to the rim. This optical zoning is a reflection of chemical zoning in the mineral. Zoning is a record of incomplete continuous reaction relations between a melt and the crystallizing solid solution as intensive parameters were changing in the magma system faster than kinetic rates could maintain equilibrium.To maintain a constant state of equilibrium between crystals and melt in the NaAlSi3O8-CaAl2Si2O8 system there is an exchange reaction between coupled ions Na+ Si4+ and Ca2+ Al3+ Exchange must occur by migration (diffusion) of these ions across the interface between the melt and already formed crystals as intensive variables change. Obviously, the larger the crystals to be modified in composition; or the more viscous the melt, which makes ions less mobile; or the faster the change in intensive variables; or combinations of these conditions, the less chance there is for equilibrium to be maintained in the system.
Zoning can be of four types: Normal or continuous zoning; reverse zoning, oscillatory zoning and patchy zoning.
Normal or continuous zoning: zoning in which the outer portions of the crystal have a lower-temperature composition than the core. Usually reflects progressive change of melt composition during growth of the crystal. Plagioclases are normally zoned from calcic cores to more sodic rims.
Reverse zoning: zoning in which the outer portions of the crystal have a higher temperature composition than the core. Usually reflects mixing between host magma and more primitive magma during crystal growth.
Patchy zoning: In many igneous rocks, the plagioclase shows patchy zoning, which consists of irregular corroded cores, the corroded portions having been filled and surrounded in crystallographic continuity by more sodic plagioclase. Several stages of patchy zoning may be present. This microstructure has been interpreted as being due to initial crystallization of relatively calcic plagioclase in a water-undersatured magma at depth, followed by decrease in confining pressure, causing resorption, owing to the fact that the melting point decrease with falling pressure in most water-deficient system. The resorption appears to be followed by new crystallization of more sodic plagioclase that is stable under the lower-pressure condition, as rims on the cores and filling of cavities in the cores, forming pseudo-inclusions of sodic in more calcic plagioclase.
Oscillatory zoning: zoning in which the composition varies cyclically from core to rim, producing concentric rings of lower and higher extinction angle and interference color. Fluctuations of the order of 30% in An content are not unusual.
Normal zoning reflects a crystal’s continuing re-equilibration to changing melt composition as the host magma evolves during the growth history of the phenocryst in a magma chamber. Explanations for oscillatory zoning, on the other hand, are necessarily more complex:
Oscillatory zoning can be explained as a crystal’s response to fluctuating external conditions. Bowen (1928), for example, attributed it to recycling of plagioclase phenocrysts through zones of cooler, more evolved melt and hotter, more primitive melt in a convecting magma chamber. Others have interpreted it as a record of a crystal’s reaction to repeated influxes of fresh magma and its mixing with more evolved resident magma. Oscillatory zoning can be explained also in terms of local effects. In this context it is important to understand that liquidus and solidus curves for pure anhydrous plagioclase (Fig. 1) are depressed by the presence of other components. Water alone can depress the curves by several hundreds of degrees, and the shape of the curves changes too.
Consider then the local environment of a crystal during magma cooling. As growth proceeds, the components not required by the plagioclase crystal increase in concentration immediately adjacent to the crystal. These components will include H20. The solidus and liquidus curves for the immediate environs of the crystal become depressed (Fig. 1), and at a fixed temperature (1400°C in Fig. 1) the crystallizing plagioclase composition shifts toward the anorthite content.
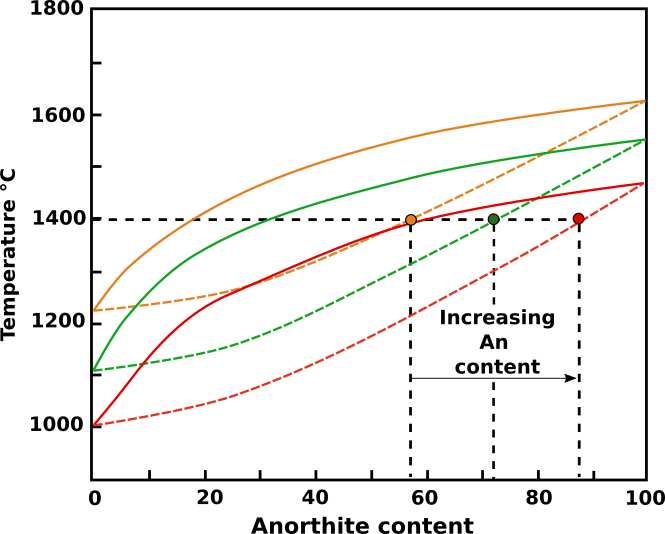
Fig.1: Rappresentazione schematica del sistema Ab-An. All’aumentare del contenuto in acqua, la composizione dei cristalli tenderà man mano a divenire più calcica. Immagine modificata da Bennett et., al (2019).
Development of Zoning
Equilibrium crystallization: In equilibrium crystallization, the crystals remain suspended in the melt, and cooling and crystallization are slow enough to allow continuous, complete reaction between crystals and melt. The early formed crystals will, on cooling, react with the melt continuously and thereby gradually change their composition. In such circumstances the end product is a homogeneous mixed crystal (solid solution) having the same composition as the initial melt.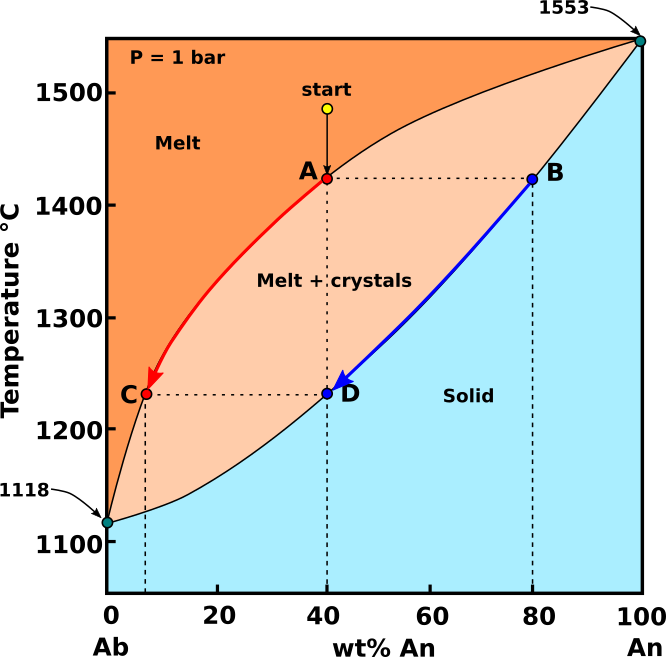
Fig.2: Diagramma Ab-An in cui è rappresentata la cristallizzazione di un ipotetico magma con composizione start (An40 - Ab60) in condizioni di cristallizzazione all’equilibrio. Modificato da Bowen, 1913.
In the system Ab-An, a cooling melt of the composition start (An40 - Ab60) will begin to crystallize at around 1470 °C. The plagioclase that crystallizes from this melt has the composition B (An80 - Ab20). As cooling proceeds, the extraction of Ca-plagioclase enriches the melt in Na. In equilibrium crystallization, plagioclase remains to react with the melts, so the crystals present become more Na-rich as well. Both the plagioclase and the melt become more Na-rich (see heavy red and blue arrow) in Fig.1. Although both plagioclase and melt progressively become more Na-rich, the bulk composition of the system does not change because the abundances of plagioclase and melt change in tandem. Plagioclase becomes more abundant and melt becomes less abundant until at 1230 °C, plagioclase, point D, matches the composition of the bulk system (An40 - Ab60). The last melt to crystallize will have a composition at C, and the solid will have a composition equal to the original starting composition.
Fractional crystallization: If the crystals are continuously removed from the melt, by sinking or some natural filtering process, reaction of crystals with the melt is prevented, and the composition of the liquid will continue to change along the liquidus curve toward the sodic feldspar component. As the liquid phase changed composition with continuing removal of crystals, the successively formed crystals would become continuously more sodic.
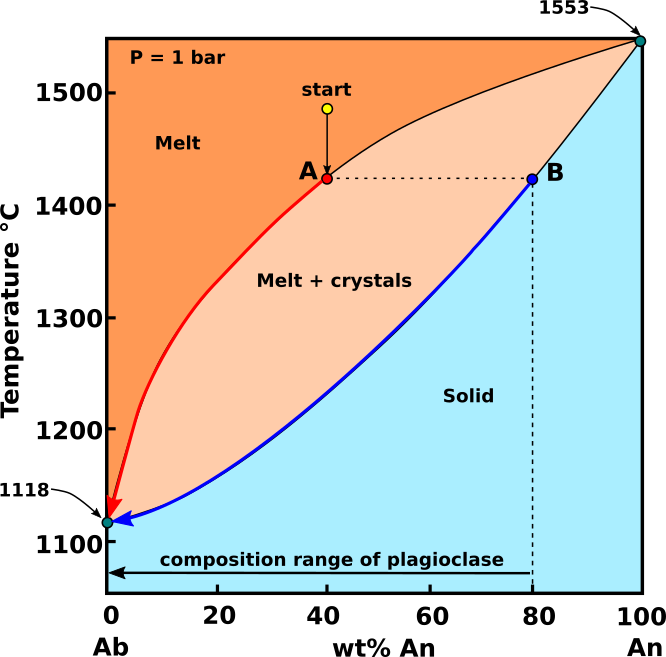
Fig.3: Ab-An phase diagram, showing the path followed by crystals and melt with composition "start", during fractional crystallization. Modified from Bowen, 1913.
If the An-Ab system undergoes fractional crystallization (Fig.3), each batch of crystallizing plagioclase is removed from reaction with the melt. As with equilibrium crystallization, the first plagioclase to crystallize from a melt with composition start (An40 - Ab60), will be a plagioclase with composition B (An80 - Ab20). Subsequent removal of the crystals will make the melt mor Na-rich. However, because the plagioclase does not react with the melt, fractional crystallization of increasingly Na-rich plagioclase causes the melt composition to move all the way to An0. After crystallization ceases, rather than having a rock with plagioclase of single composition, the rock contains plagioclase with a composition that range from B (An80 - Ab20) to An0.
Bibliography
• Cox et al. (1979): The Interpretation of Igneous Rocks, George Allen and Unwin, London.
• Howie, R. A., Zussman, J., & Deer, W. (1992). An introduction to the rock-forming minerals (p. 696). Longman.
• Le Maitre, R. W., Streckeisen, A., Zanettin, B., Le Bas, M. J., Bonin, B., Bateman, P., & Lameyre, J. (2002). Igneous rocks. A classification and glossary of terms, 2. Cambridge University Press.
• Middlemost, E. A. (1986). Magmas and magmatic rocks: an introduction to igneous petrology.
• Shelley, D. (1993). Igneous and metamorphic rocks under the microscope: classification, textures, microstructures and mineral preferred-orientations.
• Vernon, R. H. & Clarke, G. L. (2008): Principles of Metamorphic Petrology. Cambridge University Press.


.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)